Hydroxamic acid

In organic chemistry, hydroxamic acids are a class of organic compounds having a general formula
R−C(=O)−N(−OH)−R' bearing the functional group−C(=O)−N(−OH)−, where R and R' are typically organyl groups (e.g., alkyl or aryl) or hydrogen. They are amides (R−C(=O)−NH−R') wherein the nitrogen atom has a hydroxyl (−OH) substituent. They are often used as metal chelators.Common example of hydroxamic acid is aceto-N-methylhydroxamic acid (H3C−C(=O)−N(−OH)−CH3). Some uncommon examples of hydroxamic acids are formo-N-chlorohydroxamic acid (H−C(=O)−N(−OH)−Cl) and chloroformo-N-methylhydroxamic acid (Cl−C(=O)−N(−OH)−CH3).
Synthesis and reactions
Hydroxamic acids are usually prepared from either esters or acid chlorides by a reaction with hydroxylamine salts. For the synthesis of benzohydroxamic acid (C6H5−C(=O)−NH−OH orPh−C(=O)−NH−OH, where Ph is phenyl group), the overall equation is:
- C6H5−C(=O)−O−CH3 + NH2OH → C6H5−C(=O)−NH−OH + CH3OH
Hydroxamic acids can also be synthesized from aldehydes and N-sulfonylhydroxylamine via the Angeli-Rimini reaction. Alternatively, molybdenum oxide diperoxide oxidizes trimethylsilated amides to hydroxamic acids, although yields are only about 50%. In a variation on the Nef reaction, primary nitro compounds kept in an acidic solution (to minimize the nitronate tautomer) hydrolyze to a hydroxamic acid.
A well-known reaction of hydroxamic acid esters is the Lossen rearrangement.
Coordination chemistry and biochemistry
- Sample gallery
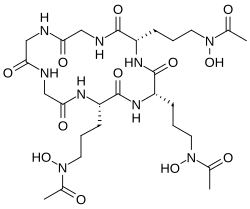
- Fe(III) complex of triacetylfusarinine
The conjugate base of hydroxamic acids forms is called a hydroxamate. Deprotonation occurs at the−N(−OH)− group, with the hydrogen atom being removed, resulting in a hydroxamate anionR−C(=O)−N(−O−)−R'. The resulting conjugate base presents the metal with an anionic, conjugated O,O chelating ligand. Many hydroxamic acids and many iron hydroxamates have been isolated from natural sources.
They function as ligands, usually for iron. Nature has evolved families of hydroxamic acids to function as iron-binding compounds (siderophores) in bacteria. They extract iron(III) from otherwise insoluble sources (rust, minerals, etc.). The resulting complexes are transported into the cell, where the iron is extracted and utilized metabolically.
Ligands derived from hydroxamic acid and thiohydroxamic acid (a hydroxamic acid where one or both oxygens in the−C(=O)−N(−OH)− functional group are replaced by sulfur) also form strong complexes with lead(II).
Other uses and occurrences
Hydroxamic acids are used extensively in flotation of rare earth minerals during the concentration and extraction of ores to be subjected to further processing.
Some hydroxamic acids (e.g. vorinostat, belinostat, panobinostat, and trichostatin A) are HDAC inhibitors with anti-cancer properties. Fosmidomycin is a natural hydroxamic acid inhibitor of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXP reductoisomerase). Hydroxamic acids have also been investigated for reprocessing of irradiated fuel.
References
Further reading
- Fouché, K. F.; H. J. le Roux; F. Phillips (June 1970). "Complex formation of Zr(IV) and Hf(IV) with hydroxamic acids in acidic solutions". Journal of Inorganic and Nuclear Chemistry. 32 (6): 1949–1962. doi:10.1016/0022-1902(70)80604-2. ISSN 0022-1902. Archived from the original on 2013-01-04. Retrieved 2009-04-24.
- Barocas, A.; F. Baroncelli; G. B. Biondi; G. Grossi (December 1966). "The complexing power of hydroxamic acids and its effect on behaviour of organic extractants in the reprocessing of irradiated fuels--II : The complexes between benzohydroxamic acid and thorium, uranium (IV) and plutonium (IV)". Journal of Inorganic and Nuclear Chemistry. 28 (12): 2961–2967. doi:10.1016/0022-1902(66)80023-4. ISSN 0022-1902. Archived from the original on 2013-01-04. Retrieved 2009-04-24.
- Baroncelli, F.; G. Grossi (May 1965). "The complexing power of hydroxamic acids and its effect on the behaviour of organic extractants in the reprocessing of irradiated fuels--I the complexes between benzohydroxamic acid and zirconium, iron (III) and uranium (VI)". Journal of Inorganic and Nuclear Chemistry. 27 (5): 1085–1092. doi:10.1016/0022-1902(65)80420-1. ISSN 0022-1902. Archived from the original on 2013-01-04. Retrieved 2009-04-24.
- Al-Jarrah, R. H.; A. R. Al-Karaghouli; S. A. Al-Assaf; N. H. Shamon (1981). "Solvent extraction of uranium and some other metal ions with 2-N-butyl-2-ethyl octanohydroxamic acid". Journal of Inorganic and Nuclear Chemistry. 43 (11): 2971–2973. doi:10.1016/0022-1902(81)80652-5. ISSN 0022-1902. Archived from the original on 2013-01-04. Retrieved 2009-04-24.
- Gopalan, Aravamudan S.; Vincent J. Huber; Orhan Zincircioglu; Paul H. Smith (1992). "Novel tetrahydroxamate chelators for actinide complexation: synthesis and binding studies". Journal of the Chemical Society, Chemical Communications (17): 1266–1268. doi:10.1039/C39920001266.
- Koshti, Nirmal; Vincent Huber; Paul Smith; Aravamudan S. Gopalan (1994-02-28). "Design and synthesis of actinide specific chelators: Synthesis of new cyclam tetrahydroxamate (CYTROX) and cyclam tetraacetonylacetone (CYTAC) chelators". Tetrahedron. 50 (9): 2657–2664. doi:10.1016/S0040-4020(01)86981-7. ISSN 0040-4020.

![Fe(III) complex of triacetylfusarinine[7]](https://upload.wikimedia.org/wikipedia/commons/thumb/5/5b/Fe%28hydroxamate%293.svg/250px-Fe%28hydroxamate%293.svg.png)