Linear chain compound
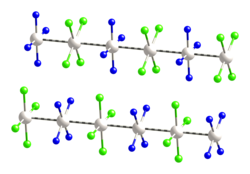
In chemistry and materials science, linear chain compounds are materials composed of one-dimensional arrays of metal-metal bonded molecules or ions. Such materials exhibit anisotropic electrical conductivity.
Examples
Many linear chain compounds feature square planar complexes. One example is
Rh(acac)(CO)2, which stack withRh···Rh distances of about 326 pm. Classic examples include Krogmann's salt and Magnus's green salt. Another example is the partially oxidized derivatives of[Pt(oxalate)2]2−. The otherwise ordinary complexIrBr(CO)3 gives an electrically conductive derivative upon oxidation, e.g., with bromine to giveIrBr1+x(CO)3−x, where x ~0.05. Related chlorides have the formulaeIrCl1+x(CO)3 andK0.6Ir(CO)2Cl2·½H2O.In contrast to linear chain compounds, extended metal atom chains (EMACs) are molecules or ions that consist of a finite, often short, linear strings of metal atoms, surrounded by organic ligands.

One group of platinum chains is based on alternating cations and anions of[Pt(CNR)4]2+ (R = iPr,c-C12H23,p-(C2H5)C6H4) and[Pt(CN)4]2−. These may be able to be used as vapochromic sensor materials, or materials which change color when exposed to different vapors.
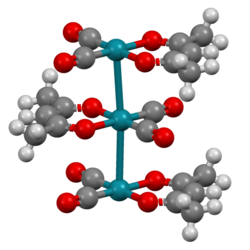
Linear chains of Pd-Pd bonds protected by a "π-electron sheath" are known.
Not only do these olefin-stabilized metal chains constitute a significant contribution to the field of organometallic chemistry, both the complex's metal atom structures and the olefin ligands themselves can conduct a current.
Methodology
Some linear chain compounds are produced or fabricated by electrocrystallization. The technique is used to obtain single crystals of low-dimensional electrical conductors.