Perkin reaction
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin in 1868 that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.

Several reviews have been written.
Reaction mechanism
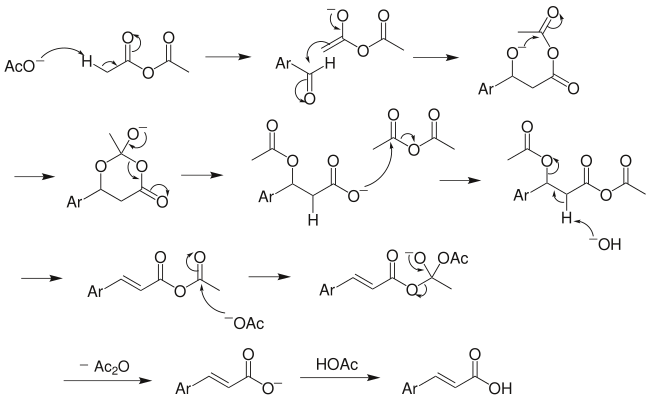
Clear from the reaction mechanism, the anhydride of aliphatic acid must contain at least 2 α-H for the reaction to occur. The above mechanism is not universally accepted, as several other versions exist, including decarboxylation without acetic group transfer.
Applications
- Salicylaldehyde converted to coumarin using acetic anhydride with acetate as base.
- cinnamic acid is prepared from benzaldehyde, again using acetic anhydride in the presence of sodium or potassium acetate.
- resveratrol (cf. fo-ti), a phytoestrogenic stilbene is yet another product of this methodology.