Pyrazole
Pyrazole is an organic compound with the formula
(CH)3N2H. It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.Properties
Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å
History
The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane.
Preparation
Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation:
Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine (Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole:
- CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O
A wide variety of pyrazoles can be made so:
Occurrence and uses

In 1959, the first natural pyrazole, 1-pyrazolyl-alanine, was isolated from seeds of watermelons.
In medicine, derivatives of pyrazole are widely used, including celecoxib and similar COX-2 inhibitors, zaleplon, betazole, and CDPPB. The pyrazole ring is found within a variety of pesticides as fungicides, insecticides and herbicides, including fenpyroximate, fipronil, tebufenpyrad and tolfenpyrad. Pyrazole moieties are listed among the highly used ring systems for small molecule drugs by the US FDA
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is used in the manufacture of six commercial fungicides which are inhibitors of succinate dehydrogenase.
Pyrazole is an inhibitor of the alcohol dehydrogenase enzyme, and, as such, is used as an adjuvant with ethanol, to induce alcohol dependency in experimental laboratory mice.
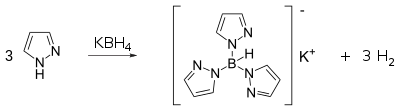
Conversion to scorpionates
Pyrazoles react with potassium borohydride to form a class of ligands known as scorpionate. Pyrazole itself reacts with potassium borohydride at high temperatures (~200 °C) to form a tridentate ligand known as Tp ligand:
See also
- 3,5-dimethylpyrazole
- Pyrazolidine, fully saturated analogue
- imidazole, structural analogue of pyrazole with two non-adjacent nitrogen atoms.
- isoxazole, another analogue, the nitrogen atom in position 1 replaced by oxygen.
References
Further reading
A. Schmidt; A. Dreger (2011). "Recent Advances in the Chemistry of Pyrazoles. Part 2. Reactions and N-Heterocyclic Carbenes of Pyrazole". Curr. Org. Chem. 15 (16): 2897–2970. doi:10.2174/138527211796378497.